De Broglie Wavelength Formula
The CODATA 2018 value for the Compton wavelength of the electron is 2426 310 238 67 73 10 12 m. De Broglie Wavelength Formula.

1 020 Me Gusta 1 Comentarios Physics Classes Joshi Physics Classes En Instagram De Broglie S Hypothesis Of Matte Particles Of Matter Hypothesis Physics
He coined the word photon for the quanta or particle of light.

. Soon scientists began to wonder if other particles could also have a dual wave-particle nature. While an electron has properties of a particle the de Broglie equation may be used to describe its wave properties. This example problem demonstrates how to find the energy of a photon from its wavelengthTo do this you need to use the wave equation to relate wavelength to frequency and Plancks equation to find the energy.
Louis Victor Pierre Raymond 7th Duc de Broglie d ə ˈ b r oʊ ɡ l i also US. 4This section contains 10. This type of problem is good practice at rearranging equations using correct units and tracking significant figures.
De-Broglie wavelength of a particle is inversely proportional to the momentum of that particular body. Find the requested quantities of a photon involved in the following hydrogen electron transitions A. Other particles have different.
Since you have posted a question with multiple sub-parts we will solve the first three sub-parts. For an electron de Broglie wavelength equation is. Please note that the formula for each calculation along with detailed calculations are available below.
Consider the wavelength of the beam of red light to be 698 nm. This example problem demonstrates how to find the wavelength of a moving electron using de Broglies equation. SECTION - B Numerical Value Type Questions.
Heat Of Fusion Formula. Where h is the Planck constant m is the particles proper mass and c is the speed of lightThe significance of this formula is shown in the derivation of the Compton shift formulaIt is equivalent to the de Broglie wavelength with. Flow Rate Formula.
The wavelength is known as the de Broglie wavelength. Calculate kinetic energy of an electron having wavelength 1nm A 21 eV B 31 eV C 15 eV D 42 eV. From the de Broglie relation we see that slowly moving electrons have a large wavelength and fast moving electrons have a short wavelength.
De Broglie wavelength λ h p Further mechanics Impulse F Δt Δp Kinetic energy of a non-relativistic particle E k p2 2m Motion in a circle v 0 ωr T 2π ω F ma mv2 r a v2 r a rω2 Centripetal force F mv2 r F mrω2 Fields Coulombs law F k Q 1 Q 2 r2 where k 1 4πε 0 Electric field strength E F Q E k Q r 2 E V d Electric potential V k Q r Capacitance C Q. 15 August 1892 19 March 1987 was a French physicist and aristocrat who made groundbreaking contributions to quantum theoryIn his 1924 PhD thesis he postulated the wave nature of electrons and suggested that all matter has wave properties. D ə b r oʊ ˈ ɡ l iː d ə ˈ b r ɔɪ French.
In old days radios contained vacuum tubes that generated and speeded up electrons. Speed Of Sound Formula. What will be the energy if the wavelength decreases to 500 nm that is if the source emits green light.
The wave which is associated with the particles that are moving are known as the matter-wave and also as the De Broglie wave. As you enter the specific factors of each resistance due to temperature calculation the Resistance Due To Temperature Calculator will automatically calculate the results and update the Physics formula elements with each element of the resistance due to temperature calculation. This formula for lambda is called the de Broglie relation and lambda is called the de Broglie wavelength of the electron.
Here h is Plancks constant m is the mass and v is the velocity of the particle. In expression of wavelength 7 e Å K the value of K is e denotes the de-Broglie wavelength of electron 1 V 2 p 3 KE 4 m Answer 1 Sol. Distance Speed Time Formula.
In laymans terms the de Broglie equation says that every moving particle microscopic or macroscopic has its own wavelength. Momentum And Its Conservation Formula. We will use the equation to find de-Broglie wavelength to find the kinetic energy of the electron with wavelength 1nm.
De-Broglie wavelength of an electron accelerated through a potential difference V is given by 1227 e Å V KV 19. Inverse Square Law Formula. De broglie wavelength 110-15 m Rest energy of proton 093810⁹ eV Q.
λ frachmv Here λ points to the wave of the electron in question. M is the mass of the electron. The significance of the de Broglie relationship is that it proved mathematically that matter can behave as a wave.
Calculate the energy of the photons emitting red light. De-Broglie Wavelength Formula Einstein proposed that any electromagnetic radiation including light which was till then considered an electromagnetic wave in fact showed particle-like nature.

De Broglie Wavelength Problems In Chemistry Chemistry Physics Problem

De Broglie Wavelength Problems In Chemistry Physical Electronics 3 Physics Chemistry Visible Light
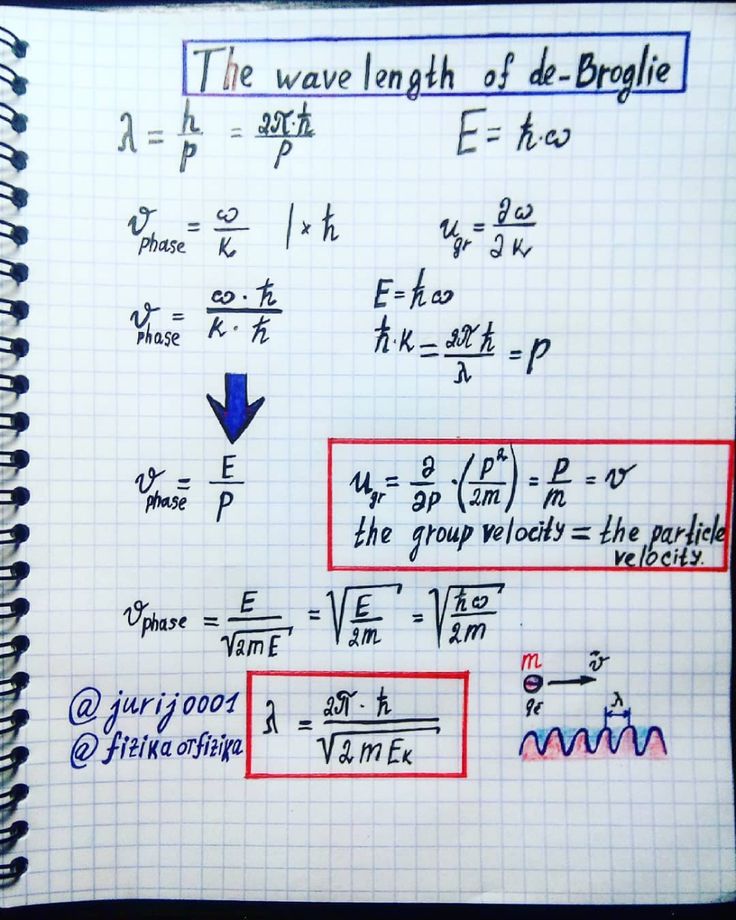
Wavelength Of De Broglie Engineering Notes Learn Physics Physics And Mathematics
Comments
Post a Comment